Seminar Abstracts
Return to seminar schedule
4/2/2025:
First Speaker: Juistina Thomas, PhD Student, Greenberg Lab, WSU Dept. of Biological Sciences
Title: Pyruvate dehydrogenase complex dysregulation links cardiolipin deficiency to metabolic defects in Barth syndrome
Abstract: Barth syndrome (BTHS) is a rare genetic disorder that causes weakness of the heart and skeletal muscles. It is caused by mutations in the TAFAZZIN gene that disrupt the remodeling of cardiolipin, a lipid essential for mitochondrial energy production. This presentation will explore how cardiolipin deficiency leads to impaired activity of the pyruvate dehydrogenase complex (PDC), an enzyme linking glycolysis to energy production. It will highlight how cardiolipin-dependent regulation of PDC contributes to the metabolic defects seen in BTHS and how this understanding may reveal new therapeutic strategies.
Second Speaker: Eseiwi Obaseki, PhD Candidate, Hariri Lab, WSU Dept. of Biological Sciences
Title: Mdm1 is required for efficient autophagy
Abstract: Efficient communication between organelles is essential for maintaining cellular homeostasis, and membrane contact sites (MCSs) play a critical role in this process. While mutations in MCS components have been linked to various diseases, the underlying mechanisms remain poorly defined. Using budding yeast, we found that the ER-vacuole tether protein Mdm1 is required for both selective and bulk autophagy. Our data show that Mdm1 facilitates autophagosome formation by possibly by promoting lipid biosynthesis, revealing a key role for MCSs in autophagy regulation.
3/19/2025:
Speaker: Durga Singer, M.A., M.D. Valerie Castle Opipari M.D. Professor of Pediatrics; Associate Professor, Departments of Pediatrics and Molecular & Integrative Physiology; Assistant Dean for Tenure Track Faculty - University of Michigan Medical School; Associate Director of Basic Research Training and Mentoring - Elizabeth Weiser Caswell Diabetes Institute
Title: Sex differences in high fat diet induced myelopoiesis and macrophage activation
Abstract: Sex differences in adiposity have long been realized but the dimorphic inflammation responses to obesogenic diets systemically and in adipose tissue have only been understood more recently. Sex differences in myelopoiesis and macrophage activation demonstrate that male animal models have more pro-inflammatory responses while females have a more regulatory inflammation response leading to preserved adipose tissue function. In addition, Dr. Singer will also discuss implications of these differences in responses to infection and describe current translational studies to understanding which adolescents are at risk for impaired metabolic health in response to obesogenic diets.
3/5/2025:
First Speaker: Andrew Butcko, PhD Candidate, Mottillo Lab, WSU Dept. of Physiology
Title: Investigating metabolic pathways impacted by PNPLA3 in brown fat – a work in progress
Abstract: Previous studies have shown that a mutation in PNPLA3 is the strongest genetic factor in causing fatty liver disease, but the primary role of PNPLA3 in non-pathological states is not well understood. Our investigation suggests that PNPLA3 plays a role in the metabolism of phospholipids, an essential component of cellular membranes. By uncovering the specific metabolic pathways impacted by PNPLA3 our work aims to shed light on its role in regulating metabolic homeostasis, with the hopes of improving treatments for fatty liver disease and other related metabolic disorders.
Second Speaker: Anuradha Yadav, PhD Candidate, Kelly Lab, WSU Dept. of Physics & Astronomy
Title: Understanding how ligands regulate protein interactions on lipid droplets by using fluorescence cross-correlation spectroscopy (FCCS)
Abstract: Lipid droplets (LDs) are central to cellular lipid metabolism and are regulated by the dynamic interplay of proteins including α/β hydrolase domain-containing protein 5 (ABHD5), perilipin 5 (PLIN5), and adipose triglyceride lipase (ATGL). Using fluorescence cross-correlation spectroscopy (FCCS), the real-time kinetics of these protein interactions have been monitored. Our findings demonstrate that SR ligands (a class of small-molecule ligands) promote the dissociation of ABHD5 from PLIN5, thereby activating ATGL and initiating lipolysis. Conversely, oleoyl coenzyme A (Oleoyl-CoA) stabilizes the ABHD5-PLIN5 complex, inhibiting lipolytic activity. This study offers novel insights into the molecular mechanisms underlying metabolic regulation and potential therapeutic interventions.
2/5/2025:
First Speaker: Shahnaz Parveen, PhD Candidate (Rumble Fellow), WSU Dept. of Physics & Astronomy
Title: The membrane biophysics of ABHD5-regulated lipolysis
Abstract: The storage and mobilization of lipids are in part regulated by alpha/beta hydrolase domain-containing 5 (ABHD5), which senses the cell’s metabolic needs and (de)activates lipid hydrolases. Despite extensive research, the mechanisms underlying ABHD5’s association with lipid droplets (LDs) remain poorly understood. My work investigates the regulatory factors that influence ABHD5 binding and localization to LD membranes, focusing on how ABHD5 distinguishes between LD monolayers and bilayers. We found that ABHD5 preferentially associates with monolayers composed of triacylglycerol (TAG) but also binds to bilayers in the presence of diacylglycerol (DAG), with DAG enhancing bilayer binding. This study sheds light on the interplay between lipid composition and ABHD5 sorting, providing insights into lipid metabolism and potential therapeutic targets for metabolic disorders.
Second Speaker: Raja Narayanasamy, Ph.D., Postdoctoral Fellow, WSU Center for Molecular Medicine and Genetics
Title: ABHD4: a new player in cardiolipin metabolism
Abstract: Cardiolipin (CL) is vital for mitochondrial function, yet the pathways that mediate CL metabolism are poorly understood. Based on its striking structural similarity with CLD1, a yeast CL lipase, we hypothesized that α/β hydrolase domain-containing protein 4 (ABHD4) is a novel mammalian CL hydrolase. We demonstrate that ABHD4 hydrolyzes CL with high selectivity in vitro, producing mono- and di-lysoCL. We discovered that ABHD4 exists as two splice variants, one of which (isoform 2) is highly expressed cells with abundant mitochondria and specifically targets the outer mitochondrial membrane. Homology modeling and mutagenesis experiments confirmed the conservation and location of the catalytic triad across ABHD4 orthologs. These findings establish ABHD4 as a key enzyme in CL metabolism and suggest an important role in mitochondrial dynamics and cell survival.
1/22/2025:
Speaker: Michael Schlame, M.D., Professor, Dept. of Anesthesiology, Perioperative Care, and Pain Medicine & Dept. of Cell Biology - New York University Grossman School of Medicine; Director of Cardiothoracic Anesthesia - NYU Langone Health
Title: A Novel Pathway to Recycle Unsaturated Lysophospholipids: Emerging Evidence for a “Too Much Food” signal
Abstract: Lysosomal phospholipid degradation produces two types of intermediates, 2-lysophospholipids with saturated fatty acids in sn-1 position and 1-lysophospholipids with unsaturated fatty acids in sn-2 position. They may either be degraded further or be reused for phospholipid synthesis. We found that the enzyme LPGAT1 recycles unsaturated 1-lysophospholipids, the more precious of the two intermediates, to regenerate cellular membranes. Purified LPGAT1 had stearoyl-CoA acyltransferase activity with strong substrate specificity for 1-lysopospholipids if they carried unsaturated chains. Substrates originated from phospholipid breakdown pathways, such as endocytosed lipoproteins. Deletion of LPGAT1 in mice increased the concentration of unsaturated lysophospholipids, altered the molecular composition of membrane phospholipids, triggered lipid droplet formation, and inhibited the regeneration of mitochondrial membranes. Thus, LPGAT1 occupies a central position in the regulation of lipid fluxes. While low LPGAT1 activity promotes the storage of lipids, high LPGAT1 activity promotes their use for membrane regeneration.
1/8/2025:
First Speaker: Alexis Wilson, PhD Candidate, WSU Dept. of Pharmacology
Title: SCD and ATF4 interplay promotes the tumor adaptive response to adipocyte-induced stress to promote prostate cancer progression in bone
Abstract: Bone metastatic prostate cancer (PCa) is an incurable disease that does not respond to standard therapeutic strategies. Bone is a hospitable environment abundant in adipocytes (fat cells), which are metabolic cells that interact with neighboring cells by secreting adipokines and lipids. We have previously determined that fat cells promote lipid uptake by PCa cells, activate tumor-promoting pathways, and play a role in chemoresistance. Our research is devoted to investigating the molecular mechanisms behind the tumor-promoting effects of fat cells to find drug targets that may have therapeutic implications for PCa. Through PCa cell-adipocyte interaction studies, we have determined that fat cell-induced stress stimulates an adaptive response in PCa cells by promoting the activation of ATF4 and SCD, factors critical for relieving stress and regulating lipid metabolism in the tumor. Overall, our findings demonstrate that this novel interplay between ATF4 and SCD pathways promotes prostate cancer progression in bone.
Second Speaker: Amit Kumar, Ph.D., Postdoctoral Fellow, WSU Dept. of Physics
Title: Understanding the Membrane Recognition Mechanism of ABHD5 Protein
Abstract: Lipid metabolism is crucial for maintaining energy balance and overall physiological health. When this process goes wrong, it can cause obesity and diabetes. ABHD5 (also called CGI-58) helps break down stored fats into fatty acids, which is important for energy use. ABHD5 plays a significant role in regulating lipid droplet (LD) dynamics, which are critical for neutral lipid storage. If ABHD5 doesn’t work properly, it can cause problems like Chanarin-Dorfman syndrome, where fat builds up in tissues. The structure of ABHD5 and how it interacts with lipid droplets is still unclear. We use MD simulations to learn how ABHD5 binds to membranes, providing insights that could lead to treatments for fat-related disorders.
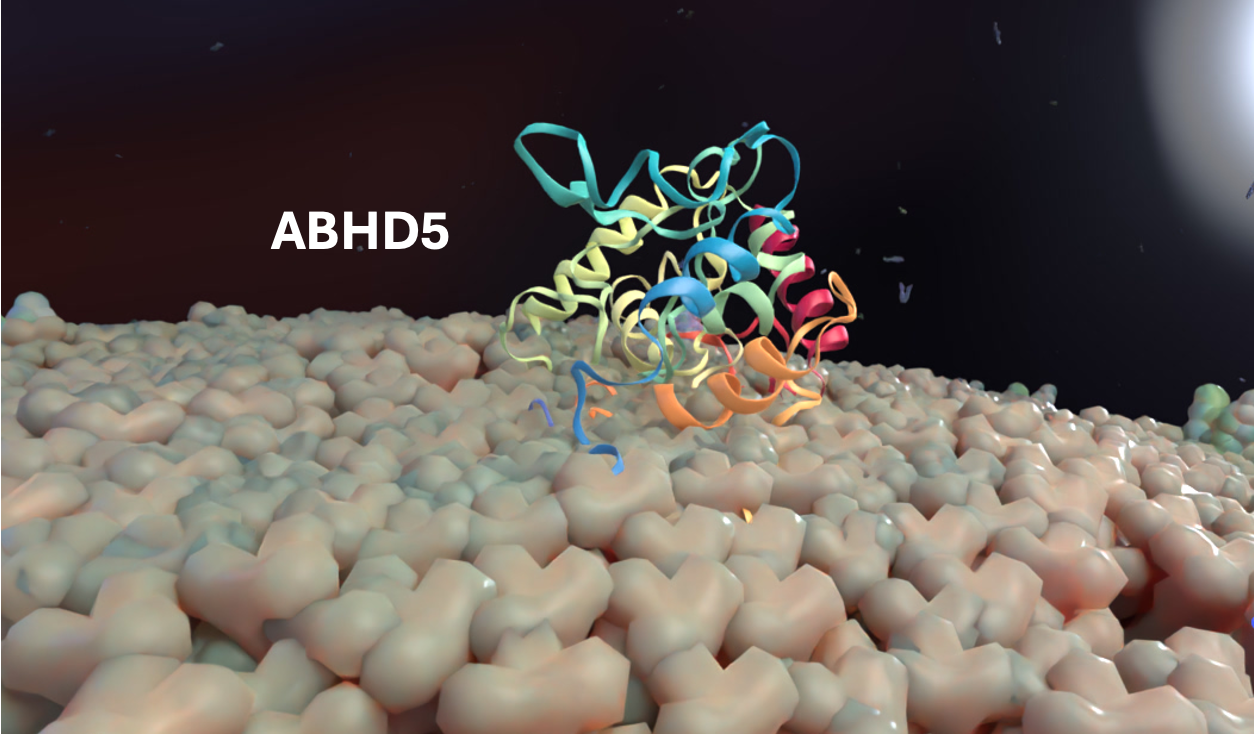
12/18/2024:
Speaker: Kymberly M. Gowdy, Ph.D., Associate Professor, Ohio State University - Dept. of Internal Medicine
Title: Dietary N3-PUFAs Modulate the Pulmonary Response to Environmental Exposures
Abstract: Inhaled environmental exposures are a substantial public health concern contributing to respiratory morbidity and mortality. Novel therapeutic strategies to limit the adverse health effects of environmental exposures is an unmet clinical need. Evidence supporting a role for diet in the response to air pollution is mounting, and n-3 polyunsaturated fatty acids (PUFAs) are a critical and potentially protective nutrient whose dietary consumption has declined with industrialization of food production. A novel paradigm has been proposed linking n-3 PUFA intake and reductions in morbidity attributable to environmental exposures. New evidence brings forth a compelling common mechanism involving cellular lipid metabolism, dietary n-3 PUFA intake, and protection against environmental pollutant induced pulmonary immunity/inflammation. This talk will provide a bench to bedside perspective of the mechanisms by which diet, and specifically N-3 PUFAs, can modulate inflammation in rodent and human models of environmental induced lung injury and inflammation.
12/4/2024:
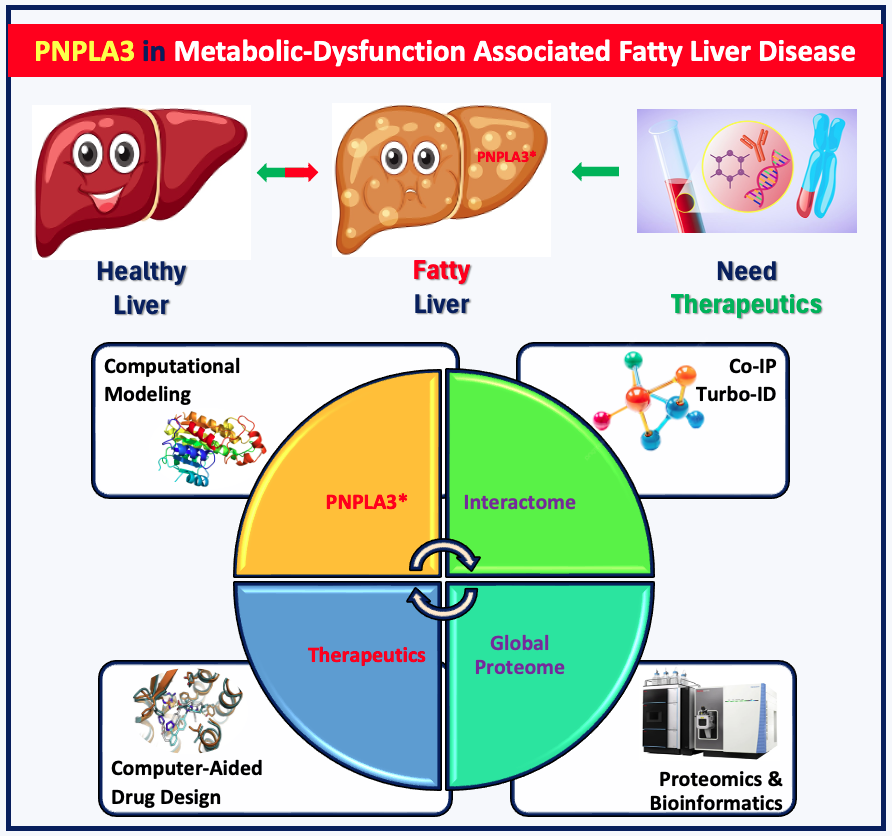
First Speaker: Arifur Rahman, PhD Candidate, WSU Dept. of Pharmaceutical Sciences
Title: Proteomic Profiling of PNPLA3 Mutants in Human Hepatocytes for Metabolic-Dysfunction Associated Fatty Liver Disease
Graphical abstract:
Second Speaker: Tyler Ralph-Epps, PhD Candidate, WSU Dept. of Biological Sciences
Title: How does cardiolipin influence mitochondrial substrate utilization?
11/20/2024:
Speaker: Itay Budin, Ph.D., Assistant Professor, University of California San Diego
Title: Phospholipid Curvature: A Biophysical Deep Dive
Abstract: Our lab investigates how – and why – cells control the composition of their lipid membranes. Lipids are traditionally challenging to study, and I will briefly present new chemical biology tools that can be used to interrogate their subcellular distributions. I will then focus on biophysical studies that seek to understand why small chemical changes to lipid chemistry are biologically important. I will show how the shape of lipid molecules – described by their spontaneous curvature – is a biophysical parameter that has driven adaptations in phospholipid chemistry. Lipid curvature is regulated in specific membranes in cells, like the inner mitochondrial membrane, where it can support function. Across organisms, lipid curvature is a property that is maintained through evolution. I will describe a wide-ranging investigation into the membranes of marine invertebrates that led us to discover how deep-sea environments act on lipid curvature and, in turn, how lipid metabolism must maintain this property.
Biography: Itay Budin is an Assistant Professor in the Departments of Chemistry & Biochemistry and Bioengineering at UC San Diego. Trained as a biophysicist, his lab investigates the interplay between lipid chemistry and cell membrane biology in a wide range of systems. Itay received his BS from Cornell University’s College of Engineering before carrying out PhD studies at Harvard University with Jack Szostak on model membranes relevant to the early evolution of cells. He then carried out postdoctoral research as a Miller Fellow at UC Berkeley, applying synthetic biology tools to lipids. He is the recipient of the Walter Shaw Young Investigator Award in lipid biology from the American Society for Biochemistry and Molecular Biology and early career awards from the National Science Foundation and Department of Energy.
11/6/2024:
First Speaker: Yaroslav Balytskyi, Ph.D., Postdoctoral Fellow, WSU Dept. of Physics
Title: Unsupervised Clustering of Docking Scores Predicts ABHD5 Ligands Activity
Abstract: We developed a model that efficiently clusters ABHD5 ligands into active and inactive categories with 92.9% accuracy using only their SMILES-based chemical structures. Notably, the model distinguishes between ligands with subtle structural differences, correctly classifying them into active or inactive groups, and provides interpretable predictions in terms of key residue interactions driving activity classification. Given ABHD5's therapeutic potential, this proof-of-concept work establishes a foundation for designing more selective drugs to modulate its activity with promising applications in treating metabolic disorders, lipid storage diseases, cancer, and inflammatory conditions.
Second Speaker: Mohamed Chakkour, PhD Candidate, WSU Dept. of Biological Sciences
Title: Phospholipids Out of Context: From Structure to Signaling
Abstract: In addition to their well-known role as structural components of cell membranes, phospholipids have been recognized as critical players in various cellular processes. This presentation explores the non-canonical roles of some phospholipids, highlighting their involvement in signaling pathways, gene expression regulation, enzymatic activation, and neuronal messaging. By examining these functions, I aim to shed light on the expanding roles of phospholipids beyond ordinary membrane precursors and delve into their importance in cellular communication and homeostasis.
10/16/2024:
Speaker: Mike Lange, Ph.D., Postdoctoral Fellow, University of California, Berkeley - Department of Nutritional Sciences & Toxicology, Department of Molecular & Cell Biology
Title: Protecting Stored Lipids From Damage: Ferroptosis Suppressor Protein 1 Prevents Lipid Peroxidation In Lipid Droplets
Abstract: Lipids are crucial for cellular function but their susceptibility to damage such as peroxidation, can have adverse effects on cellular wellbeing. Cells deploy diverse lipid quality control mechanisms to prevent the buildup of toxic oxidized lipids. Loss of lipid quality control, particularly on phospholipids in the plasma membrane, results in the catastrophic loss of membrane integrity leading to ferroptosis – a lipid peroxidation-dependent cell death modality.
Beyond membranes, lipids can form lipid droplets, organelles that consist of hydrophobic lipids such as triglycerides and steryl esters. Lipid droplets facilitate regulated lipid storage and release, thereby ensuring safe lipid metabolism in tissues such as adipose and liver, particularly under conditions of lipid overload. Dysregulation of lipid droplet levels is associated with pathological conditions such as obesity or fatty liver disease. Despite their importance, the existence of lipid quality control mechanisms that safeguard lipid droplets from damage remains uncertain. Furthermore, it is unknown what the consequences of lipid droplet damage are on cellular health.
We have identified the first lipid droplet quality control system executed by ferroptosis suppressor protein 1 (FSP1), a protein previously recognized as one of the essential membrane lipid quality control systems. Using chemical biology tools in genome-edited cancer cell lines, we found FSP1 bound to lipid droplets protects cells from lipid peroxidation-induced death. In vitro reconstitution biochemistry, combined with lipidomics and epilipidomics analyses, shows that lipid droplet resident FSP1 acts as a coenzyme Q10 reductase, locally generating the antioxidant ubiquinol, thus preventing lipid droplet damage. This marks the first description of a lipid droplet quality control system, and we are currently investigating how this novel pathway is involved in the disease pathogenesis of dysregulated metabolically active tissues such as adipose tissue during obesity.
10/2/2024:
First speaker: Laimar Garmo, PhD Candidate, WSU Dept. of Pharmacology
Title: Dysregulation of bone homeostasis through exposure to PFAS: the impact on bone marrow adipogenesis and osteoclastogenesis
Abstract: Per- and polyfluoroalkyl substances (PFAS) are environmental contaminants that tend to accumulate in bone. Peroxisome proliferator-activated receptors (PPARs), notably PPARa and PPARg, play essential roles in lipid metabolism, adipogenesis, osteoclastogenesis and bone turnover. PFAS exposure has been linked with PPAR activation, modulation of lipid metabolism, and reduced bone mineral density. However, little is known about the potential impact of PFAS exposure on bone homeostasis. We hypothesized that PFAS accumulating in bone disrupt bone homeostasis through the activation of PPARs. Using in vitro and in vivo approaches, we have identified two specific PFAS compounds, PFHxS and GenX, as a key modulators of PPAR-mediated marrow adipogenesis and osteoclastogenesis. Collectively, our data suggest that PFAS accumulating in bone have a potential to dysregulate bone homeostasis through PPAR-mediated mechanisms.
Second speaker: Aaron Lotvola, PhD Candidate, WSU Dept. of Oncology
Title: Regulation of c-Myc protein expression by ABHD5 in Prostate Cancer Cells
Abstract: Purpose: The survival outlook of prostate cancer patients remains poor and incurable when castration-resistant cells (hormone-resistant) adapt, evade, and proliferate to last-line therapy which ultimately results into castration-resistant prostate cancer (CRPC). To improve patient outcomes, the identification of key factors for resistance are essential to provide the foundation for the next generation of advanced therapeutics in CRPC. Metabolic rewiring, a hallmark of cancer in PCa, is enhanced in CRPC and ensures that resistant cells meet their anabolic demand for macromolecules, such as the shunting of glucose carbon to sustain the synthesis of nucleotides used in DNA replication, and energy to support continuous cellular proliferation. The lipolytic gene and co-activator for lipid turnover, ABHD5 (αβ-hydrolase domain containing 5), is also a novel tumor suppressor in CRPC. However, the mechanism behind ABHD5-dependent tumor suppression is not well understood. Our earlier results demonstrated that ABHD5-mediated lipolysis acts as a functional barrier suppressing the anabolic signaling of cancer cells. Mechanistically, ABHD5 triggers futile cycling of triglyceride hydrolysis and re-synthesis, resulting in AMP accumulation, AMPK activation, and mTORC1 inactivation. Methods and Results: RNA-seq analysis of ABHD5 overexpression revealed that without affecting mRNA levels, the MYC oncogene is a gene target of ABHD5 and strongly downregulated. Gene-set enrichment analysis (GSEA) indicated that Myc-target genes such as PHGDH and SHMT2, which are vital enzymes in serine-biosynthesis that support oncogenic activities, are also downregulated in presence of ABHD5 overexpression. Conclusion: Defining an ABHD5/cMYC/PHGDH regulatory axis will establish a new paradigm within cancer metabolism and potentially innovate cancer intervention by directly targeting cMYC via ABHD5’s regulatory cascade.